Abstract
Prolonged antibiotic administration has been associated with bone marrow suppression in approximately 10-15% of patients. The mechanisms that underlie the association between prolonged antibiotic therapy and bone marrow suppression, including neutropenia, remain poorly understood. Our recent studies in murine models demonstrated that prolonged broad-spectrum antibiotics suppress hematopoiesis by depletion of the intestinal microbiota. Furthermore, oral administration of the microbial metabolites nucleotide-binding oligomerization domain-containing protein 1 ligand (NOD1L) and desaminotyrosine (DAT) was sufficient to restore normal hematopoiesis in antibiotic-treated mice. Additionally, Type I IFN signaling in hematopoietic cells was noted to be critical for the intestinal microbiota to promote hematopoiesis, as administration of IFNα during antibiotic therapy rescued normal hematopoiesis. These findings support a paradigm in which products of the intestinal microbiota enter the bloodstream and travel to the bone marrow where they promote the production of growth cytokines that support normal hematopoiesis.
To elucidate whether changes in the microbiome similar to those observed in mice also occur in humans treated with prolonged courses of antibiotics, we carried out a multicenter non-treatment, non-intervention study. We enrolled patients aged 0-17 years receiving long-term (>2 weeks) intravenous (IV) or oral (PO) antibiotic courses across two tertiary children's hospitals. We excluded patients with history of abnormal blood counts, bone marrow failure, gastrointestinal infection / syndromes / abnormalities, immunodeficiency, malignancy, severe malnutrition, use of prophylactic antibiotics or use of antibiotics immediately before admission. Patients noted to develop neutropenia while receiving antibiotics were enrolled in the retrospective arm. A stool sample was collected at 2 timepoints: at the start and at the completion of antibiotics for the prospective arm of the study; at the onset of neutropenia while on antibiotics and at resolution of neutropenia after completion of antibiotics for the retrospective arm. Here, we report an interim analysis performed for an initial cohort of 5 patients with antibiotic-associated neutropenia and 10 controls matched for age and race/ethnicity.
For clinical data analysis, we assessed continuous variables using parametric and non-parametric tests when appropriate while using χ2 or Fisher's exact tests for categorical variables. Stool samples were sent for 16s rDNA sequencing to determine whether there are significant changes in the composition (individual microbial taxa) and diversity (both alpha and beta) of the gut microbiome between groups; as well as for untargeted metabolomic profiling to assess whether changes in the relative abundance of specific microbiome-related metabolites, including but not limited to NOD1L and DAT, are associated with the development of neutropenia with prolonged antibiotic treatment. Analyses of these data are currently ongoing.
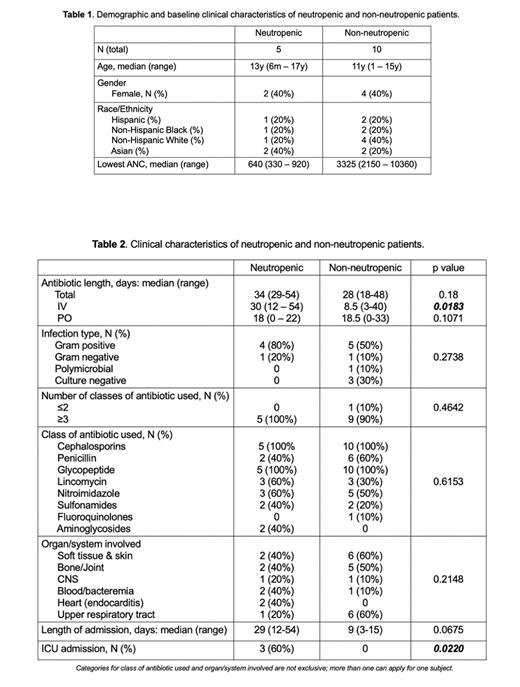
Neutropenia occurred in 9.4% of our prospective patient samples (3 out of 32 patients). Median age was 13y (range 6 mo. - 17y) for the neutropenic group and 11y (range 1-15y) for the control group. Forty percent of patients were female in both groups (Table 1). Total length of IV antibiotic and ICU admission were positively correlated with neutropenia in this cohort (Table 2).
To our knowledge, this is the first clinical study linking changes in the intestinal microbiota with antibiotic associated neutropenia. The incidence of antibiotic-associated neutropenia was comparable to what has been previously reported. The analysis of stool samples from this cohort through 16sDNA sequencing and untargeted metabolomic profiling will outline specific changes in microbiome load, diversity, as well as specific taxa and metabolites potentially related to the development of neutropenia. Altogether, we hope to utilize an improved understanding of the molecular pathways, mediators and receptors by which the microbiome sustains normal hematopoiesis to prevent and treat antibiotic-associated bone marrow suppression.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal